17+ Chapter 4 Review Arrangement Of Electrons In Atoms Answer Key
Subtract this number from the number of valence electrons for the neutral atom. Panpsychism in the History of Western Philosophy.

Technical Proposal For The Design Construction Gsi
So this combination may occur either by sharing of electrons.

. Molecular Orbital theory or MOT is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Mass number Number of protons Number of neutrons 4 5 9. The chemical bond formed as a result of sharing of electrons among atoms is known as a covalent bond.
Now each Cl atom has seven electrons and the Br atom has seven electrons. 1091 The best writer. Atomic number Number of protons or number of electrons 4.
I Write the molecular formula of the compounds formed by these elements individually with hydrogen. NCERT Solutions Class 11 Chemistry Chapter 2 Free PDF Download. It is the bonding properties of carbon atoms that are responsible for its important role.
The arrangement given has corresponding five 1o carbon. Ii Which of these compounds will have the highest dipole moment. The correct answer is c.
Our professional team of writers ensures top-quality custom essay writing services. Typically the tautomers of a compound exist together in equilibrium and easily interchange. 7 7 0 All atoms in BrCl 3 have a formal charge of zero and the sum of the formal charges totals zero as it must in a neutral.
A single bond is formed between two atoms when two electrons are shared between them ie one electron from each participating atom. It is depicted by a single line between the two atoms. Therefore it can form four covalent bonds with other atoms or molecules.
Therefore both atoms share three electrons each and form a triple bond. The atomic number and mass number of sodium are 11 and 23 respectively. For instance all organisms are made up of cells that process hereditary information encoded in genes which can be transmitted to future generationsAnother major theme is evolution which explains the unity and diversity.
At least 1 number 1 uppercase and 1 lowercase letter. Person who is exactly at the middle is J. This gives the formal charge.
I The molecular formula of the compounds are as follows. Electron density distributions in space and energies eg. One of the first Presocratic philosophers of ancient Greece Thales c.
Browse our listings to find jobs in Germany for expats including jobs for English speakers or those in your native language. E A F J H D K. 624545 BCE deployed an analogical argument for the attribution of mind that tends towards panpsychism.
Comprehensive student-friendly answers are provided according to the latest CBSE Syllabus 2022-23 to all the in-text and exercise. The atom of an element is made up of 4 protons 5 neutrons and 4 electrons. The atoms lose their electrons more easily.
7 7 0 Cl. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. These orbitals have different shapes eg.
Due to this arrangement in Molecular Orbital Theory electrons associated with different nuclei can be found in different atomic orbitalsIn molecular orbital theory the electrons present in a molecule are not. Of atomic number no. Not based on your username or email address.
The very large difference in electronegativity between the H atom 21 and the atom to which it is bonded 40 for an F atom 35 for an O atom or 30 for a N atom combined with the very small size of a H atom and the relatively small sizes of F O or N atoms leads to highly concentrated partial charges with these atoms. Download Free PDF View PDF. Single Double and Triple Bonds and Their Strengths.
Carbon contains four electrons in its outer shell. The simplest organic carbon molecule is methane CH 4 in which four hydrogen atoms bind to a carbon atom. 2s is lower energy than 2p.
For example calcium is a group 2 element whose neutral atoms have 20 electrons and a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. A The arrangement from left to right. B An atom with electronic configuration 2 8 7 would be chemically similar to F 9.
Biology is the scientific study of life. Get all these features for 6577 FREE. Must contain at least 4 different symbols.
C 4 H 10 O can be represented as ethoxyethane C 2 H 5 OC 2 H 5 and methoxy-propane CH 3 OC 3 H 7. It can also be used as a good metaphor for this lessons concepts involving atoms. Kimia Dasar Untuk Civil Engineering.
These NCERT Solutions aim to provide students with comprehensive answers to all questions asked in Chapter 2 of the NCERT Class 11 Chemistry. Well the obvious answer is it is made of atoms which contain electrons. NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties are provided on this page for CBSE Class 11 students.
A tautomer of a compound refers to the isomer of the compound which only differs in the position of protons and electrons. Clear indications of panpsychist doctrines are evident in early Greek thought. The chapter briefly discusses the arrangement of elements in increasing order of their atomic.
NCERT Solutions for Class 10 Maths Chapter 4. It is a natural science with a broad scope but has several unifying themes that tie it together as a single coherent field. Download Free PDF.
Q-2 Write Lewis dot symbols for atoms of the following elements. S p d f and so on are the names given to the orbitals that hold the electrons in atoms. Therefore 7 m 3 would equal 7 10 3 L which is close to the answer.
Elements X Y and Z have 4 5 and 7 valence electrons respectively. We Offer the Custom Writing Service with 3 Key Benefits. In the alkane H 3 C CH 2 CCH 3 2 CH 2 CHCH 3 2 identify 1 2 3 carbon atoms and give the number of H atoms bonded to each one of these.
But enough of that smarty-pants. We strive to ensure that every paper is. When a Ca atom loses both of its valence electrons the result is a cation with 18 electrons a 2 charge and an electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.
Download Delhi 2020-21 NTSE Stage 1 Answer Key Question Paper With Solutions and use it to enhance your preparation for the exam. The n number determines how many of the subshells make up the shell. THE STUDY OF CHANGE Problem Categories.
Primary carbon atoms 1 o. NCERT Solutions Class 11 Chemistry Chapter 3 Free PDF Download. The NCERT Solutions for Class 12 Physics Chapter 4 Moving Charges and Magnetism is given here for the benefit of CBSE Class 12 Science students.
In Chapter 4 NCERT Solutions for Class 12 Physics students will learn the concepts of Moving Charges and MagnetismAlso this chapter covered several. 31. 1s is lower energy than 2s which is lower energy than 3s.
Carbon atoms bonded to a single atom of carbon are called primary atoms of carbon. What are its atomic number and mass number. NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atom are provided on this page as a free source of educational content for Class 11 students.
Therefore the atomic number is 17. NCERT Solutions for Class 10 Maths Chapter 5. Also an ionic bond is formed as a result of sharing of electrons among atoms.
NCERT Solutions for Class 12 Physics Chapter 4 Free PDF Download.

13 Electrons In Atoms
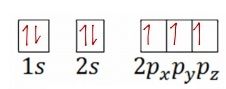
Chapter 4 Test Review Arrangement Of Electron In Atoms Flashcards Cram Com

Electrons In Atoms 5 North Plainfield School District Guset User Flip Pdf Anyflip

Chem12 C0500 Ctbs Name Date Class Electrons In Atoms Chapter Test B A Matching Match Each Term In Column B With The Correct Description In Column A Course Hero

Chapter 4 Study Guide Review Pdf Name Class Date Chapter 4 Review Arrangement Of Electrons In Atoms Section 1 Short Answer Answer The Course Hero

Group 17 Elements Halogen Family Properties Trends Uses
![]()
Cell Biology Schemes And Mind Maps Cell Biology Docsity
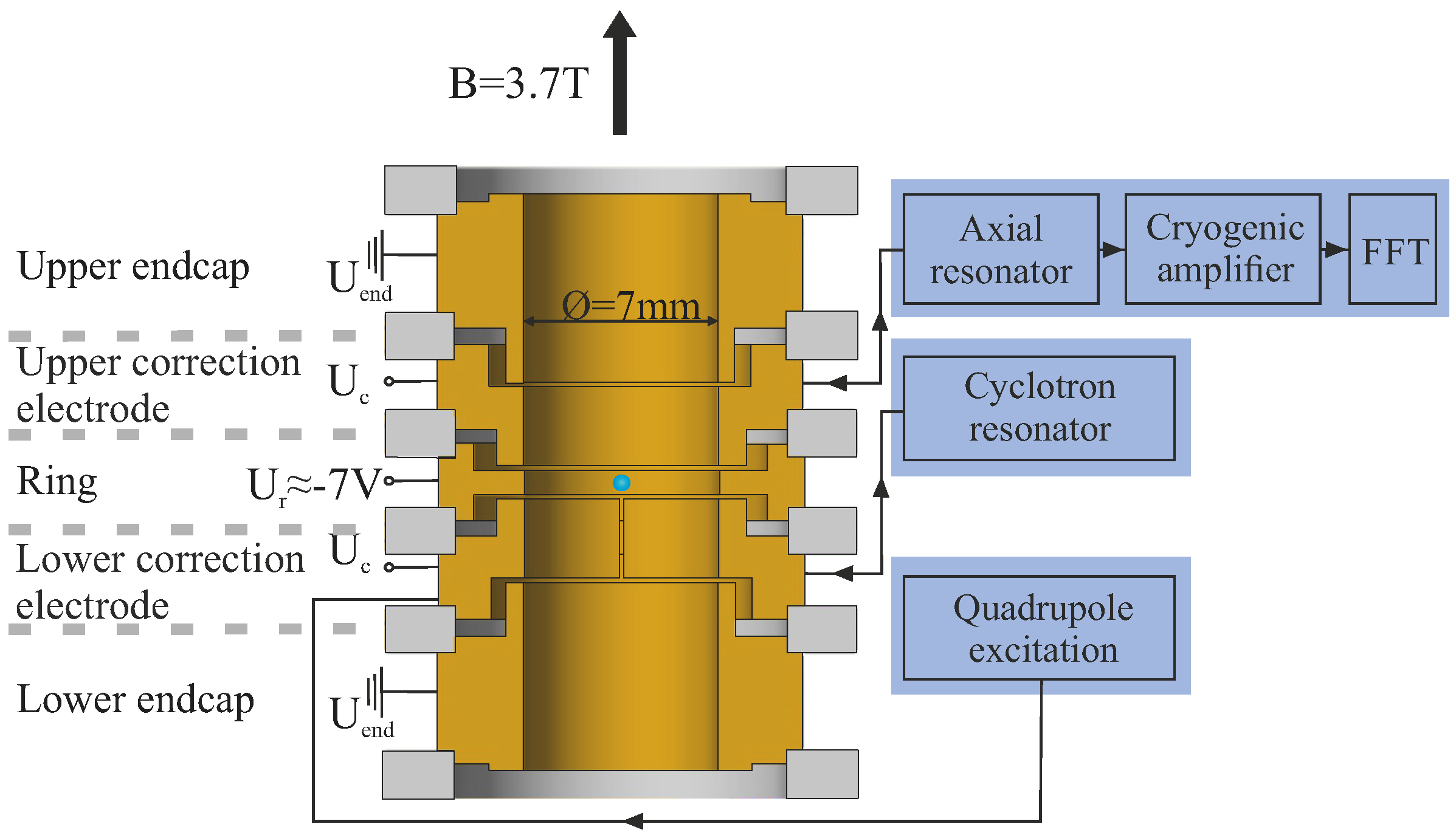
Atoms Free Full Text High Precision Measurements Of The Bound Electron S Magnetic Moment

Polymorphism In Atomically Precise Cu23 Nanocluster Incorporating Tetrahedral Cu4 0 Kernel Journal Of The American Chemical Society

Roadmap On Photonic Electronic And Atomic Collision Physics Iii Heavy Particles With Zero To Relativistic Speeds Iopscience

Sec Filing Roivant Sciences Ltd

Chapter 4 Arrangement Of Electrons In Atoms Ppt Video Online Download
Atomic Structure
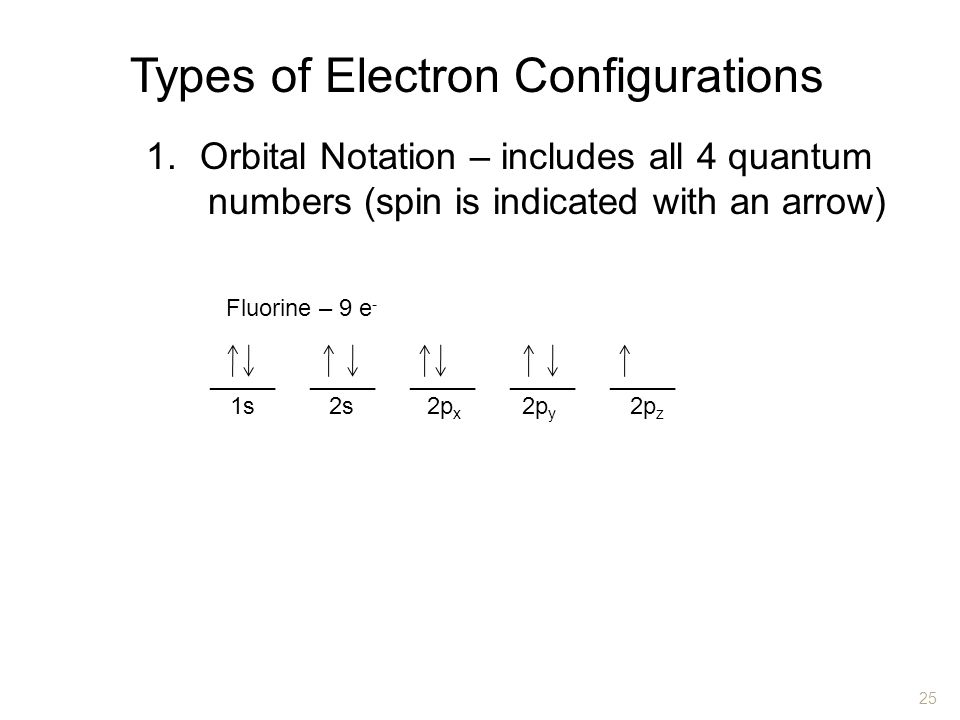
Chapter 4 Arrangement Of Electrons In Atoms Ppt Video Online Download

Pdf Can Kelly S Triads Be Used To Elicit Aspects Of Chemistry Students Conceptual Frameworks Keith S Taber Academia Edu
Chapter 4 Arrangement Of Electrons In Atoms Ppt Video Online Download

Pdf Symmetry Restoration In Mean Field Approaches